Heparan sulfate diversity and inflammatory response
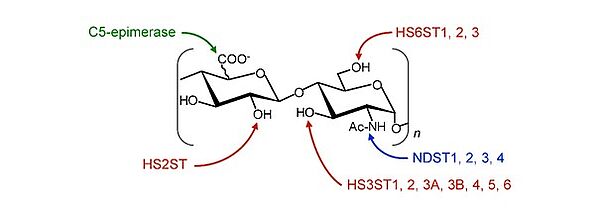
Glycosaminoglycans are sulfated polysaccharides found in abundance on the surface of cells and in extracellular matrices. They are synthesized in the form of non-sulfated polymers, the basic unit of which is a disaccharide formed from a glucuronic acid or a galactose linked to an osamine, glucosamine or galactosamine. These polymers then undergo the coordinated and sequential actions of several sulfotransferases on specific positions of the disaccharide units. Within this family, the heparan sulfates (HS) are the members with the greatest structural and functional diversity. Maturation of the precursor [GlcUA-GlcNAc]n involves a GlcUA/IdoUA epimerase and 15 sulfotransferases, which can sulfate GlcNs on C3 and/or C6 amine and hydroxyl groups, and C2 uronic acids. Once synthesized, HS can undergo a final structural modification, catalyzed by secreted 6-O-endosulfatases, the Sulfs. The majority of HS modification enzymes are represented by several isoenzymes, which possess subtle differences in substrate specificity. Moreover, since the set of modifications does not occur uniformly on the same chain, the result is a heterogeneous organization, where highly sulfated domains alternate with low-modified domains. The structural diversity of HS directly influences the functions of their numerous protein partners, including growth factors, cytokines, proteases, membrane receptors or extracellular matrix components. Thus, interactions with HS modulate the bioavailability of many cellular mediators, influence cell adhesion and migration mechanisms and promote the formation of signaling complexes.
Numerous studies have highlighted the involvement of HS in the regulation of many pathophysiological processes. Moreover, the structure of HS, and thus their interaction properties, is highly variable depending on the cell type and can be profoundly remodeled in response to changes in the tissue environment. However, the mechanisms that regulate the expression and activity of HS maturation enzymes remain poorly understood. In this context, the work of the team focuses on the mechanisms of regulation of HS-modifying enzymes and on the impact of structural remodeling of these molecules in different models of normal and pathological inflammatory responses. In particular, we are currently focusing our work on the role of 3-O-sulfation of HS in the communication between cancer cells and the immune system, and on the consequences of Sulf action in neurodegenerative processes. This work should show the importance of these HS-modifying enzymes as targets in the development of new therapeutic approaches.