Regulation of molecular and cellular mechanisms by O-GlcNAcylation
The team "Regulation of molecular and cellular mechanisms by O-GlcNAcylation" focuses its research activities on the biological functions played by the nutrient-sensitive modification O-GlcNAcylation.
State of the art
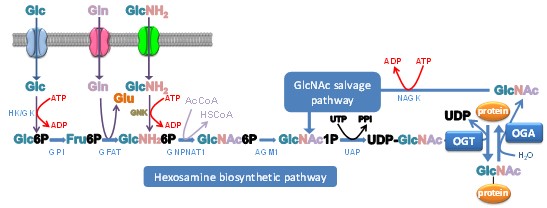
O-GlcNAcylation is a post-translational modification (PTM) belonging to the large group of glycosylations. Unlike N-, O- and C-glycosylations, O-GlcNAcylation of proteins is confined to the cytosolic, nuclear and mitochondrial compartments. O-GlcNAcylation is highly dynamic, as is phosphorylation, with which it can either act in concert or compete for the same or adjacent serine/threonine residues. There is increasing evidence that crosstalk also exists with other PTMs (e.g. acetylation, methylation, ubiquitination, proteolysis...). These interactions offer theoretically infinite combinations of PTMs on protein isoforms in proteomes. The diversity of O-GlcNAcylated proteins, the dynamics of O-GlcNAcylation and its interaction with other PTMs make this glycosylation a regulator of most, if not all, basic and fundamental cellular processes such as cell signaling, cell cycle and apoptosis; specifically, at the molecular level, O-GlcNAcylation interferes with transcription, protein synthesis, proteasomal degradation, and cytoskeleton organization. Addition and removal of the GlcNAc residue is mediated by O-GlcNAc transferase (OGT), which uses the nutrient scavenger UDP-GlcNAc as a sugar donor, and O-GlcNAcase (OGA) respectively. The level of UDP-GlcNAc, supplied by the hexosamine biosynthetic pathway (HBP), is closely correlated with the nutrient status of the cell since many metabolic pathways are required for nucleotide-sugar biosynthesis (Fig. 1). Therefore, because of its crucial position, it has been suggested that O-GlcNAcylation regulates cellular metabolism and functions in a nutrient-dependent manner.
Ongoing projects
O-GlcNAcylation and colorectal cancer
This project focuses on the role of the function of metabolic and nutritional disorders in the emergence of colorectal cancer (CRC) from O-GlcNAcylation. CRC is a leading cause of cancer mortality and morbidity and is often associated with metabolic disorders (obesity, diabetes...). Widely spread in Western societies, these two groups of pathologies are closely linked. Previous work by our team has shown that O-GlcNAcylation is increased in colon cancer cell lines and tissues and modifies key proteins involved in cancer processes.
Resistance to chemotherapy
Some of our studies concern the drug resistance of tumor cells which remains a major challenge for the efficacy of curative treatments. Several epidemiological studies have linked altered glycosylations to drug resistance mechanisms in colorectal cancer in different ways: decreased drug uptake and metabolism, strain acquisition or resistance to apoptosis. Our team has recently focused on resistance to 5-FU, a well-established chemotherapy drug used in combination with other molecules, and will next investigate resistance to FOLFOX induced by increased O-GlcNAcylation.
Development of a CRC diagnostic tool
Current CRC screening tests are unreliable and give false-positive results in 9 out of 10 cases. Extracellular vesicles (EVs) are present in some biological fluids (blood, urine, stool...) and are involved in multiple physiological and pathological processes such as CRC. Our recent data show that CRC cells produce EVs, contain proteins involved in the biological pathways of O-GlcNAcylation and the level of O-GlcNAc is increased in these CRC EVs. In addition, we have recently developed and validated a protocol to isolate EVs from stool samples (dog and human). Analysis of a first cohort of OC detector positive patients revealed a completely new signature that could be used in future CRC detection. Our data provide new insights into the field of EV glycosylation and highlight the importance of O-GlcNAcylation for potentially diagnosing CRC.
Control of complex glycosylation and vesicular trafficking by O-GlcNAcylation - focus on sialylation patterns
In line with previous work that sought to understand the relationship between complex glycosylations (N- and O-Glycans, glycolipids) and O-GlcNAcylation, part of our activities showed that reduction of OGT activity alters glycosylation of LAMP2 and TGN46, markers of the lysosome and Golgi apparatus, respectively. Thus, the idea that O-GlcNAcylation affects complex glycosylations may find part of its answer in the control of vesicular trafficking and microtubule dynamics. These analyses should also establish a link with autophagic flux. In this same theme, in collaboration with the "Chemical Glycobiology" team led by Prof. Christophe Biot (specialist in bioorthogonal chemistry), we are working to decipher how sialylation patterns can be modified by the dynamics of O-GlcNAcylation.
O-GlcNAcylated FASN as a putative driver of carcinogenesis
FASN (Fatty acid synthase) is involved in a wide variety of biological processes, including cell proliferation, and is most highly expressed in tumor cells. We have recently demonstrated that FASN interacts with OGT and is therefore O-GlcNAcylated. We have also mapped several putative O-GlcNAcylation sites on the lipogenic enzyme, but their individual roles remain unknown. FASN mutants with potential O-GlcNAcylation were generated to validate, or invalidate, these different sites. Now, the expression level, subcellular distribution, and enzymatic activity of each mutant will be assessed, and the interactome of each mutant will be explored. Xenographs in immonodeficient mice will be undertaken to assess the oncogenic potential of FASN in its O-GlcNAcylated form.
Monitoring of O-GlcNAcylated proteins in living cells
In collaboration with Prof. Christophe Biot (group "Chemical Glycobiology") and Dr. Corentin Spriet, from the PLBS platform, our objective is to dissect the role of O-GlcNAcylation on the proto-oncoprotein -catenin. This project based on the expansion of the genetic code through the incorporation of unnatural amino acids (UAA) combines molecular biology, chemical biology and ex vivo imaging to specifically visualize the O-GlcNAc/phospho isoforms of -catenin in living cells. We have previously combined the chemical reporter strategy with fluorescently labeled proteins (GFP proteins); although this strategy allows tracking of O-GlcNAcylated forms of -catenin in cells, it lacks flexibility and does not allow tracking of a specific O-GlcNAc residue of the protein in live cells. Therefore, we now propose to exploit the incorporation of UAA, such an approach should be transposable to any type of PTM in any protein.
Investigation of O-GlcNAcylation in transcriptional processes
The TATA-Box binding protein (TBP) is a universally conserved platform for the assembly of PICs (pre-initiation complexes), with TBP being a key player in the dynamic regulation of transcription initiation. We have previously shown that TBP is dynamically modified by O-GlcNAcylation at T114, T126 and S158. Specific O-GlcNAcylation of TBP at T114 is involved in the formation of the B-TFIID complex, resulting in dynamic association of TBP to chromatin and redistribution of the TBP promoter. Glycosylation of TBP at T114 regulates a set of genes involved in lipid metabolism. Yet, the primary roles of O-GlcNAcylation at T126 and S158 remain unknown. Nevertheless, TBP-specific O-GlcNAcylation may have a profound impact on the transcriptome and physiology of cells. We propose to document the specific function of these residues, using combined transcriptomics and proteomics approaches in CRISPR-modified cell lines, as well as structural conformation and DNA bending activity studies. The phenotypes of CRISPR-modified cell lines will be further investigated to link the role of each TBP O-GlcNAcylation site on cellular physiology to the underlying molecular mechanism.