GLYCOSTRESS
Recherche
Numerous epidemiological observations in pregnant women have shown a correlation between the stresses experienced during gestation and the consequences on the offspring: miscarriages, premature births, low body weight (Seckl, 2004). This led to the development of the concept of Developmental Origin of Health and Diseases (DOHaD). Since research on humans is difficult to implement, we have animal models that allow us to further develop this concept. More specifically, we want to study how exposure to a harmful environment during early life can program the predisposition of the offspring to pathologies likely to occur throughout life and even to be transmitted through generations.
The perinatal stress model in rats, developed by Prof. Maccari in 1995, consists in subjecting pregnant rats to restraint stress. The animals born from stressed mothers (PRS, Prenatal Restraint Stress) will present an anxious/depressive phenotype (Maccari et al., 1995) characterized by :
- Neuroendocrine dysregulations, particularly in the hypothalamic-pituitary-adrenal axis
- Alterations of circadian rhythms
- Alterations in behavior
We have also been able to demonstrate a transgenerational effect of stress. Indeed, the vulnerability to the development of stress-related pathologies can be programmed through maternal behavior. Therefore, we measured the maternal behavior of both dams directly exposed to repeated episodes of restraint stress during gestation (referred to here as "grandmothers") and their female offspring (referred to here as "mothers", once the latter produce offspring). In the "grandmothers", gestational stress significantly reduces maternal care. In the male offspring of these "grandmothers", we were able to show that perinatal restraint stress induces long-term biochemical and behavioral changes that lead to the expression of an anxiety/depressive type phenotype. Interestingly, most of the behavioral and neurobiological alterations associated with prenatal restraint stress persist to the second generation (here in the offspring of the "mothers"), despite the fact that these male rats were not subjected to any stress in utero. Furthermore, the maternal behavior of their mothers (female offspring of the stressed "grandmothers") is only slightly affected while these rats no longer show an anxiety-like profile.
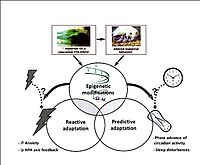
The mechanisms through which these adverse experiences are transmitted from generation to generation are not yet clear and cannot be explained solely by alterations in maternal behavior. Thus, other mechanisms may be actively involved. Indeed, in the central nervous system, perinatal stress induces epigenetic modifications, i.e. related to transmissible changes in gene expression without affecting the DNA sequence (see Figure 1). These epigenetic processes are potentially reversible, showing that our data can open new perspectives for psychiatric research and thus give a new approach to the obsolete definition of irreversibility of psychiatric disorders.
The work that we carry out in the Structural and Functional Glycobiology Unit is based on the following research axes
- Epigenetic programming of glutamatergic synapses in the ventral hippocampus correlates with an anxiety/depression-like phenotype in PRS rats
- Epigenetic programming of the dopaminergic system in PRS rats: role in the development of extrapyramidal disorders during aging
- Involvement of the oxytocinergic and dopaminergic system in the programming effects of perinatal stress on an anxious/depressive phenotype as well as on drug vulnerability in PRS rats
Members
Pr Stefania Maccari, Pr Sara Morley Fletcher, Dr Hammou Bouwalerh, Dr Gilles Van Camp