Molecular mechanisms of N-glycosylation and associated diseases
Our team is interested in the mechanisms of fine regulation of the different steps of glycosylation, in the endoplasmic reticulum (ER) and the Golgi, concerning more particularly vesicular trafficking and ionic homeostasis via the study of human glycopathologies.
The wide spectrum of glycans carried by glycoconjugates (glycoproteins, proteoglycans and glycolipids) contributes to the diversity and adaptability of cells, both in their functions and in their interaction capacities. This glycan heterogeneity is generated almost exclusively within the secretory pathway, the ER and Golgi apparatus, by the action of a battery of specific glycosidases and glycosyltransferases. Any dysfunction in the synthesis of these structures is at the origin of rare genetic and metabolic diseases grouped for the most part under the term "Congenital Glycosylation Disorders" (CGD) affecting multiple organs and almost always the brain. The originality of our work was to consider that the genetic defects found in CGDs do not only affect the enzymes involved in the maturation of glycans, but also certain molecular actors regulating their activities.
Our research is both translational in nature, aiming to identify and characterize unresolved cases of GDC and to search for therapies for these patients, and fundamental in nature, aiming to characterize and study the function of the different molecular actors identified and involved in the regulatory mechanisms. Our work is at the origin of therapeutic leads based on manganese and galactose for the treatment of certain CDG patients.
Our team has many national and international collaborations on the themes developed below. We also have a close partnership with the team of Pr. G Matthijs (KU Leven) with whom we have obtained the label "International Associated Laboratory" (LIA CNRS/FWO).
To answer our questions, we use in all our works biochemical and molecular approaches, sophisticated imaging as well as structural analysis (mass spectrometry/radioactive metabolic labelling). These different methods are applied to all the different cellular models at our disposal (patient cells, isogenic KO cells for the genes of interest, yeast cells).
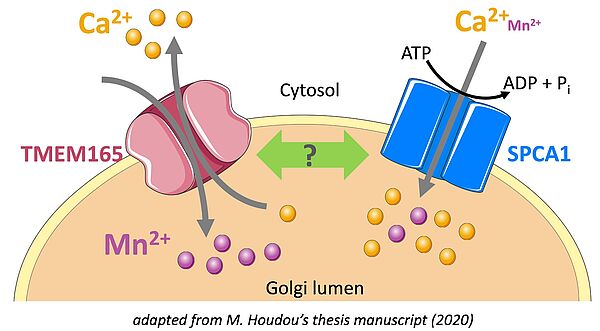
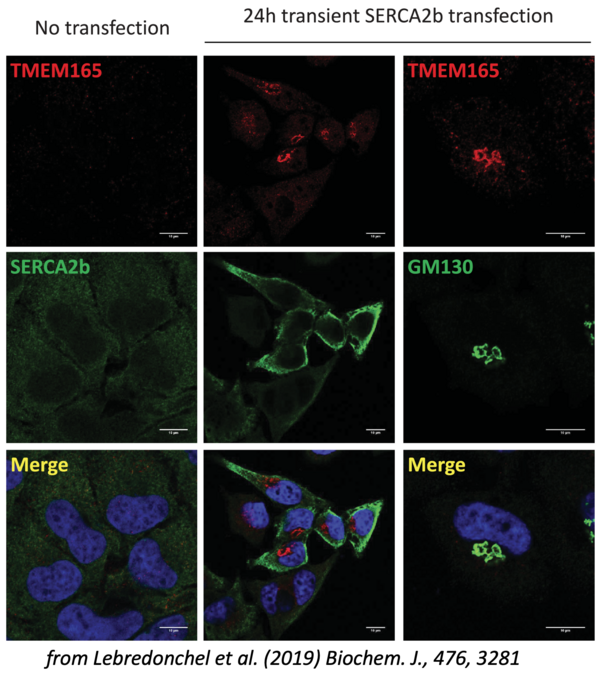
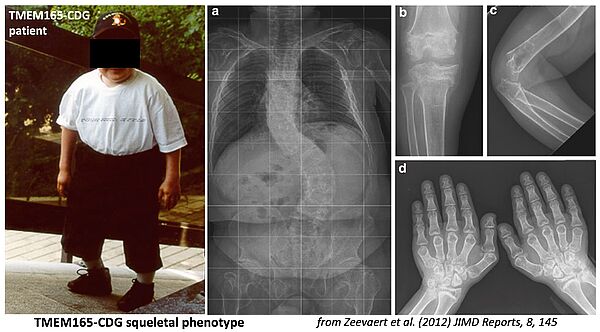
The importance of Ca2+ and Mn2+ homeostasis in the glycosylation processes of the secretory pathway is the central research theme of the team. The characterization by the team as early as 2012 of CDG patients deficient in the TMEM165 protein, mainly affected by mental retardation and skeletal dysplasia, has opened a formidable field of investigation on this molecule which turns out to be a key membrane transporter of Mn2+ in the Golgi where this ion plays a role of cofactor for many glycosylation enzymes. Interestingly, our results suggest that Mn2+ and Ca2+ homeostasis in the Golgi, governed mainly by TMEM165 and SPCA1, a transporter functionally linked to TMEM165, play a crucial role in glycosylation processes.
In connection with these studies, the team is also interested in the role of SLC10A7, which was found to be deficient in CDG patients with a skeletal dysplasia phenotype very similar to that of TMEM165-CDG. In collaboration with Prof. Valérie Cormier Daire (Imagine, Paris), we aim to understand the function of SLC10A7 in glycosylation.
Finally, the team is interested in the importance of the metabolism of free oligosaccharides (fOS) released during the process of N-glycosylation of proteins, and more particularly in the activity of the cytosolic mannosidase MAN2C1 in charge of their catabolism. Interestingly, MAN2C1 deficient patients with brain malformations and intellectual disability have been recently characterized. This discovery opens an important field of investigation to understand the role of fOS and MAN2C1 in neuronal development.